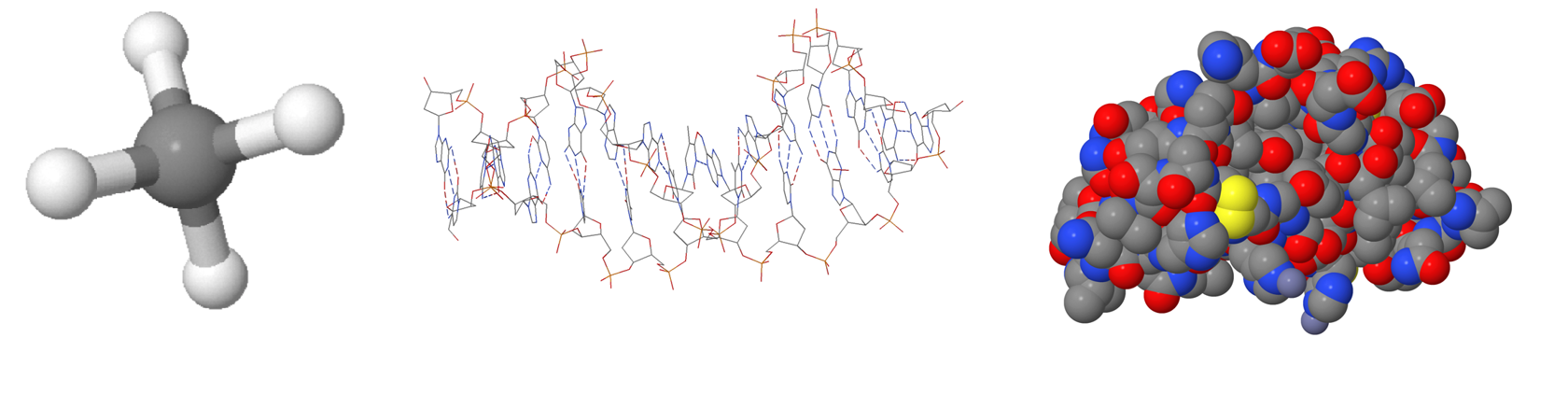
Atomic and molecular models are used to visualise the invisible world
Atoms[term: atom – Atomi on pienin rakenneosanen, josta aine rakentuu kemiallisesti.] and molecules[term: molecule – A molecule is an electrically neutral group formed by two or more covalently bonded atoms. A compound formed of molecules is called a molecular compound.] are so small that they cannot be seen with the naked eye. For this reason, molecules are depicted with models. We need different models because each type of model provides different information. The aim is to highlight the details that we are investigating. The ball-and-stick model is a typical molecular model. It provides information about atoms, their position, and the type of molecular bond. A skeletal formula only draws the bonds between atoms. The type of atoms must be deduced by the reader. A calotte model provides information about the size and geometric shape of a molecule. Sometimes molecules are drawn as a 2D structural formula. The 2D model corresponds more poorly to reality than an equivalent 3D model but may be visually easier to perceive.
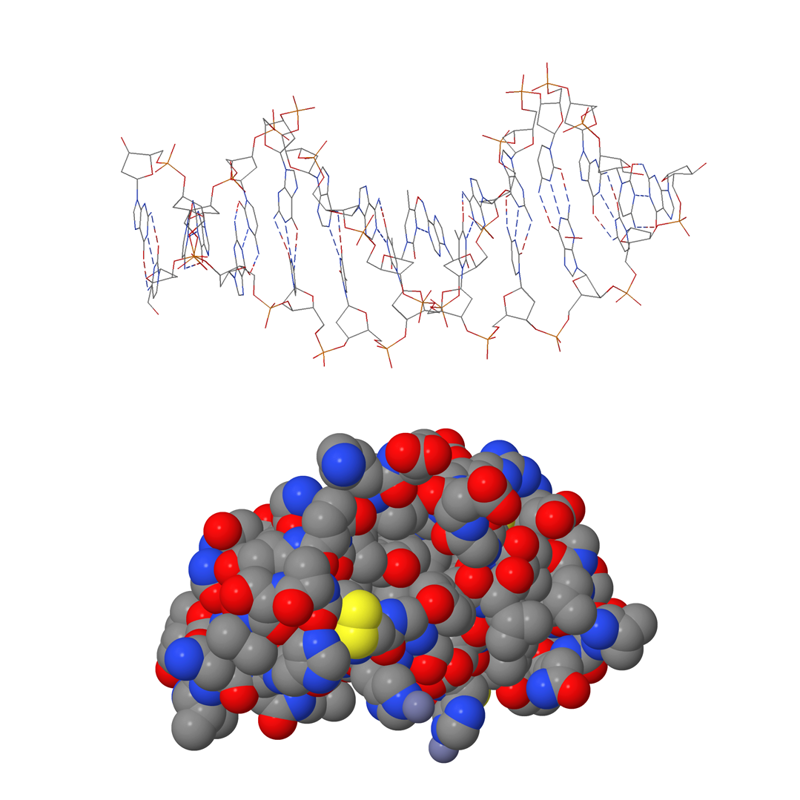
The most common molecular models
In a calotte model, atoms and their positions are represented by different coloured spheres that are embedded into one another. The atoms are represented using different colours to help identify them more easily. In reality, atoms have no colour at all. Atoms of different elements are shown using different-coloured spheres. The size of the sphere is based on computational chemistry.
In a ball-and-stick model, the balls are the same size as in the calotte model, but the distance between the atoms is greater. The rods that connect the atoms represent the electronic interactions, or bonds, between the atoms. The direction, length, and angles of the rods are based on computational chemistry. A ball-and-stick model shows the molecule’s three-dimensional structure more clearly than a calotte model.
A structural formula[term: structural formula – A structural formula describes how the atoms in a molecule are bonded to one another and the molecule’s spatial structure.] gives the type and quantity of atoms in a molecule, the type of bonds between the atoms, and the geometry of the structure. Atoms are denoted using their chemical symbols and the bonds between the atoms are shown with lines. The angles between the bond lines may be real or, for drawing-technical reasons, simplified and approximate.
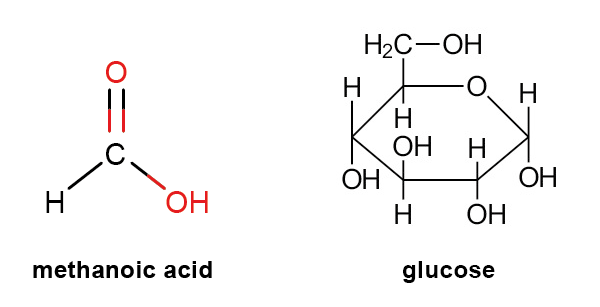
A condensed structural formula[term: condensed structural formula – A condensed structural formula is a structural formula which omits some bonds.] depicts the structure of a molecule to the same accuracy as a structural formula, but some of the bond lines are not marked. Ethanol, for example, is represented as CH3 – CH2 – OH or even more concisely as CH3CH2OH.
A skeletal formula[term: skeletal formula – A skeletal formula is a simplified structural formula in which the carbon frame is depicted as a line and only functional groups are labelled with chemical symbols.] is the simplest way to represent structural formulae. The chemical symbols of carbon and hydrogen atoms are not marked. Carbon atoms are positioned at the ends of the lines, at corners, and at branches. Every such point also implies the maximum number of hydrogen atoms that can bond to the carbon atom in question. Foreign atoms and functional groups attached to hydrocarbon chains are denoted explicitly using their chemical symbols, as are hydrogen atoms bonded to atoms other than carbon atoms.
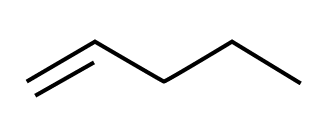
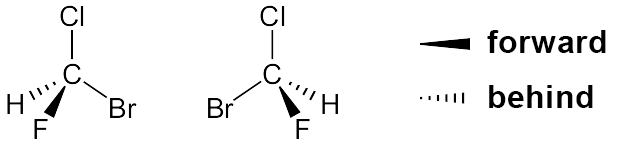
A three-dimensional structural formula is a more visual form of the structural formula. It represents the atoms’ key positions in a way that corresponds to ball-and-stick and calotte models. Three-dimensional structural formulae are used when we want to highlight a compound’s geometry in the structural formula. This is particularly significant in structural or constitutional isomerism and in spatial isomerism or stereoisomerism.
An empirical formula denotes the chemical proportions of elements in a compound. For example, the empirical formula of table salt is NaCl, which means that sodium and chloride occur in the compound in a ratio of 1:1. In glucose C6H12O6, carbon, hydrogen and oxygen occur in a ratio of 1:2:1, so its empirical formula is (CH2O)6. Sometimes a compound’s empirical formula is the same as its molecular formula. For example, H2O is both the empirical and molecular formula for water.
Subscript numbers in empirical formulae denote the ratios between quantities of elements in a mole of a compound. Different compounds can have the same empirical formula but different molecular formulae. For example, formaldehyde CH2O and glucose (CH2O)x have the same empirical formula, but in the glucose molecule, the actual quantities[term: amount of substance – The amount of substance is an SI base unit. Its symbol is n and its unit is the mole, mol.] are six times those found in formaldehyde (CH2O)6. The empirical formula can be used to determine the mass ratios and mass percentages.
Measurements tell us the type of element and their relative quantities, but not the positions of the atoms or the types of bonds. For example, the empirical formula for ethane C2H6 is CH3 or, more precisely, (CH3)x. When we find the value of x, we can solve the compound’s precise empirical, molecular, and structural formula.
A molecular formula gives the simple numbers and types of atoms contained in a compound, and possibly also their functional group. For example, the molecular formula for ethanol is C2H5OH, which highlights the OH group in the molecule. The molecular formula for glucose is C6H12O6, but this does not indicate that there are many OH groups in the compound.
Formulae of ionic compounds denote the charge of a particle. Individual atoms, groups of atoms, and molecules can occur as charged ions. For example, the formula for a water molecule is H2O, but when ionised, it becomes either an oxonium ion H3O+ or a hydroxide ion OH–.
Many other kinds of formulae are used to denote electron[term: electron – An electron is a negatively charged particle that orbits the atomic nucleus.] structures. In a Lewis structure, valence electrons[term: valence electron – Valence electrons are located in an element’s highest energy level. They determine the chemical properties of an atom (e.g. reactivity).] are denoted as dots next to the element’s chemical symbol. Electrons that form pairs are denoted with pairs of dots.
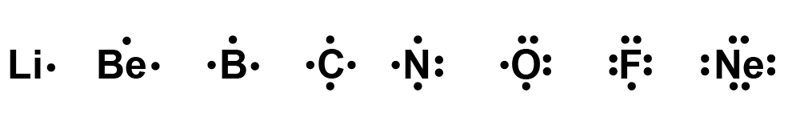
An electrostatic potential map illustrates the distribution of electric charges in the different parts of a molecule. Electrostatic potential maps are based on computational chemistry.
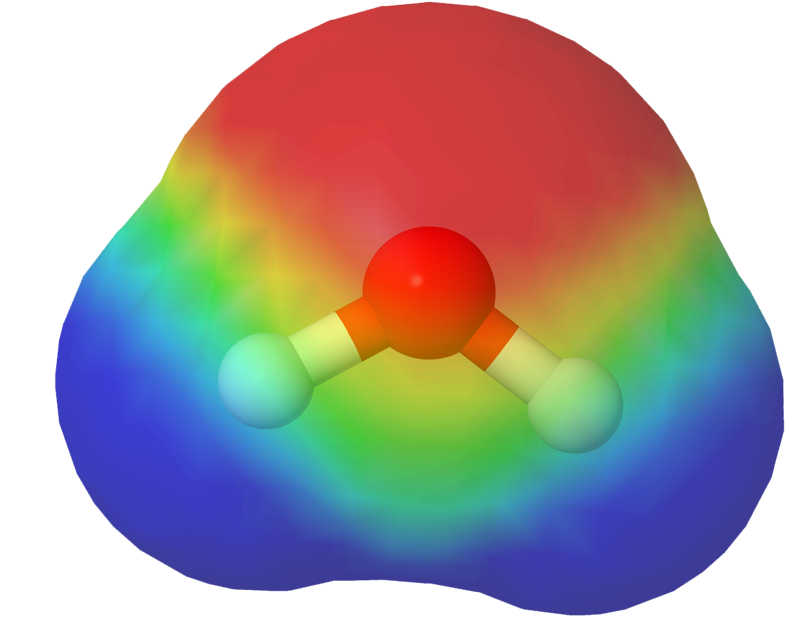
Which model should I choose?
The situation determines which model is most suitable. Choosing the right model is one of the professional skills of a chemist. No model completely corresponds to reality, because the molecule’s shape changes due to thermal vibrations.
MarvinSketch file: methanoic_acid.mrv
Lattice structures are used to denote how materials pack together
The types of lattice that determine a material’s structure are an ionic lattice, a covalent lattice, an atomic lattice, a metallic lattice and a molecular lattice. The type of lattice is determined by the type of bond holding the material together. Different lattice structures give materials different physical and chemical properties. For example, materials with ionic, covalent, and atomic lattices are hard with high melting points. The hardness, melting points and boiling points of materials with metallic lattices vary greatly. Materials with a molecular lattice are soft with low melting points.
An ionic lattice is based on ionic bonds. If ionic bonds are broken, the material melts. When the material cools, it solidifies, and the lattice reforms. A covalent lattice and atomic lattice are based on covalent bonds. For example, in silicon dioxide, each silicon and oxygen atom is covalently bonded to other atoms surrounding them. The structure continues in all directions, and it is not possible to distinguish individual SiO2 molecules – all bond lengths[term: bond length – Electrons and nuclei with like charges repel one another. The distance between the nuclei caused by attractive and repulsive forces is known as the bond length.] (Si–O) are the same. Diamond, on the other hand, comprises an extremely hard atomic lattice made of carbon atoms. Each carbon atom is covalently bonded to four other carbon atoms. A metallic lattice is based on a metallic bond. Particular properties of a metallic lattice include the free movement of electrons, which makes metals able to conduct electricity. A molecular lattice is based on weak bonds between molecules. For example, ice keeps its shape thanks to the hydrogen bonds in the water molecule. The hexagonal structure of the crystal is determined by the angles of the bond between oxygen and hydrogen atoms in the water molecule.
Comparing lattice structures
Atomic lattice | Non-polar lattice | Polar lattice | Ionic lattice | Metallic lattice | |
Solid structure | 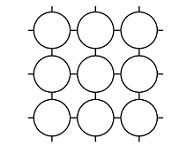 | 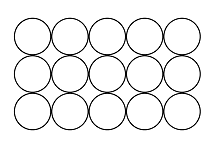 | 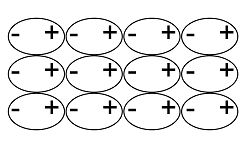 | 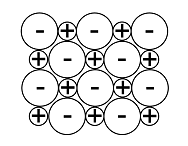 | 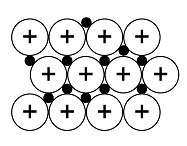 |
Structural components | atoms | non-polar molecules | polar molecules | positive and negative ions | metal ions and bonding electrons |
Bonds between components | covalent bond | dispersion force | dipole-dipole bond | ionic bond | metallic bond |
Elements | usually elements of group 14 | non-metallic and non-metallic | usually non-metallic and non-metallic | metallic and non-metallic | metallic |
Example substances | Si, C, SiC | O2, PH3 | H2O, NH3 | NaCl, MgF2 | Mg, Cu |
Electric conductivity | insulant | insulant | usually insulant, sometimes conductor | insulant (in solid form), conductor (when melted or dissolved in water) | conductor |
Solubility | non-soluble | soluble in non-polar substances | soluble in water | soluble in water | non-soluble |
Hardness | very hard | easily evaporating | hard but brittle | hard but brittle | malleable |
Summary
- We need different 3D models because each type of model provides different information. The aim is to highlight the details that we are investigating.
- A structural formula gives the type and quantity of atoms in a molecule, the type of bonds between the atoms, and the geometry of the structure.
- A condensed structural formula depicts the structure of a molecule to the same accuracy as a structural formula, but some of the bond lines are not marked.
- An empirical formula denotes the chemical proportions of elements in a compound. For example, the empirical formula of table salt is NaCl, which means that sodium and chloride occur in the compound in a ratio of 1:1.
- A molecular formula gives the simple numbers and types of atoms contained in a compound, and possibly also their functional group.