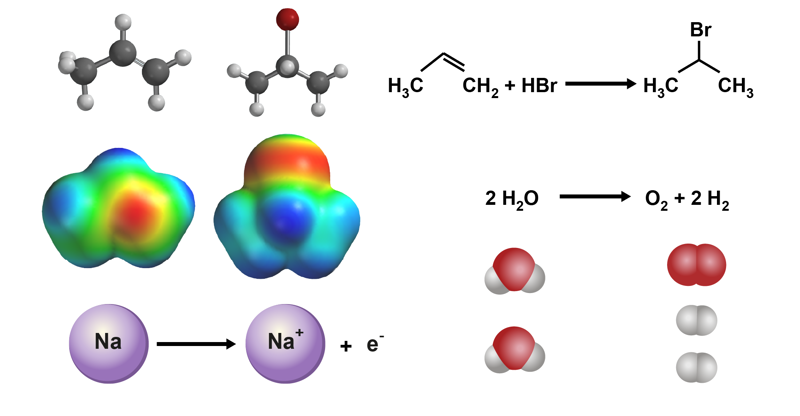
Chemical reactions

Chemical reactions are the most important area of study in chemistry. Understanding, learning and making use of chemical reactions is what makes chemistry a science. Many other scientific fields also make use of chemical reactions.
Examples of the uses of chemical reactions
- Using chemical reactions as efficiently as possible is the foundation of the chemical industry.
- Chemical reactions are used to make medicine and cosmetics.
- Cooking and nutrient storage are based on chemical reactions. The chemistry of cooking even has its own scientific field, molecular gastronomy.
- The physiology of people and animals is based on chemical reactions, and so are many functions of the ecosystem. For example, think about the cycle of different substances (e.g. water, carbon and nitrogen), the atmosphere or something as basic as a mosquito bite.
Image gallery: Chemical reactions in the environment
The key characteristic of a chemical reaction
The key characteristic of a chemical reaction is a change in a substance’s structure. The changes we can observe with our senses are the result of changes on an atomic level. When observing the atoms and electrons, the sub-micro level, we can see how the electron distribution in the atoms and molecules changes.
During a reaction, the bonds between the reactant atoms are weakened, and new bonds are formed as the atoms become rearranged. The change always moves towards an equilibrium or a more stable structure. There are also energy changes during the reaction, which can be observed as thermal and light phenomena. In addition, the reaction always takes a certain amount of time.
When does a reaction occur?
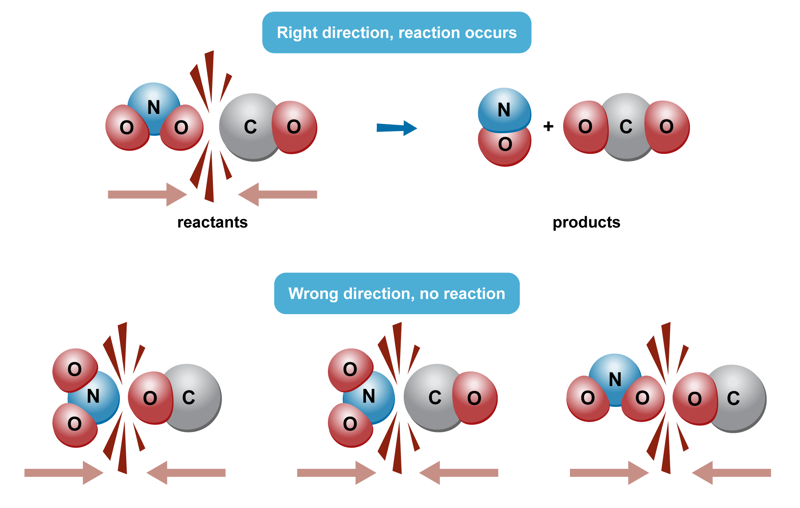
Reactants will create reaction products only when structural components that can react collide with one another at the right speeds and from the right directions. In addition to reactive substances, a chemical reaction requires the correct external conditions. Depending on the situation, a reaction may require a specific temperature, amount of radiation, pressure, concentration of substance or some other specific factor. There can be one or several variables for conditions.
As an example, hydrogen and oxygen molecules are constantly colliding with one another at room temperature, but there are not a significant number of collisions that result in a reaction. When the kinetic energy of the gases is increased by heating them, there are more favourable collisions that lead to a chemical reaction.
Review the basic concepts of chemistry
The basic concepts of chemistry
Basic concept | Law or model |
A substance or energy cannot be created from nothing or destroyed. | Principle of mass conservation |
All matter consists of small structural components, including atoms, molecules and ions. | 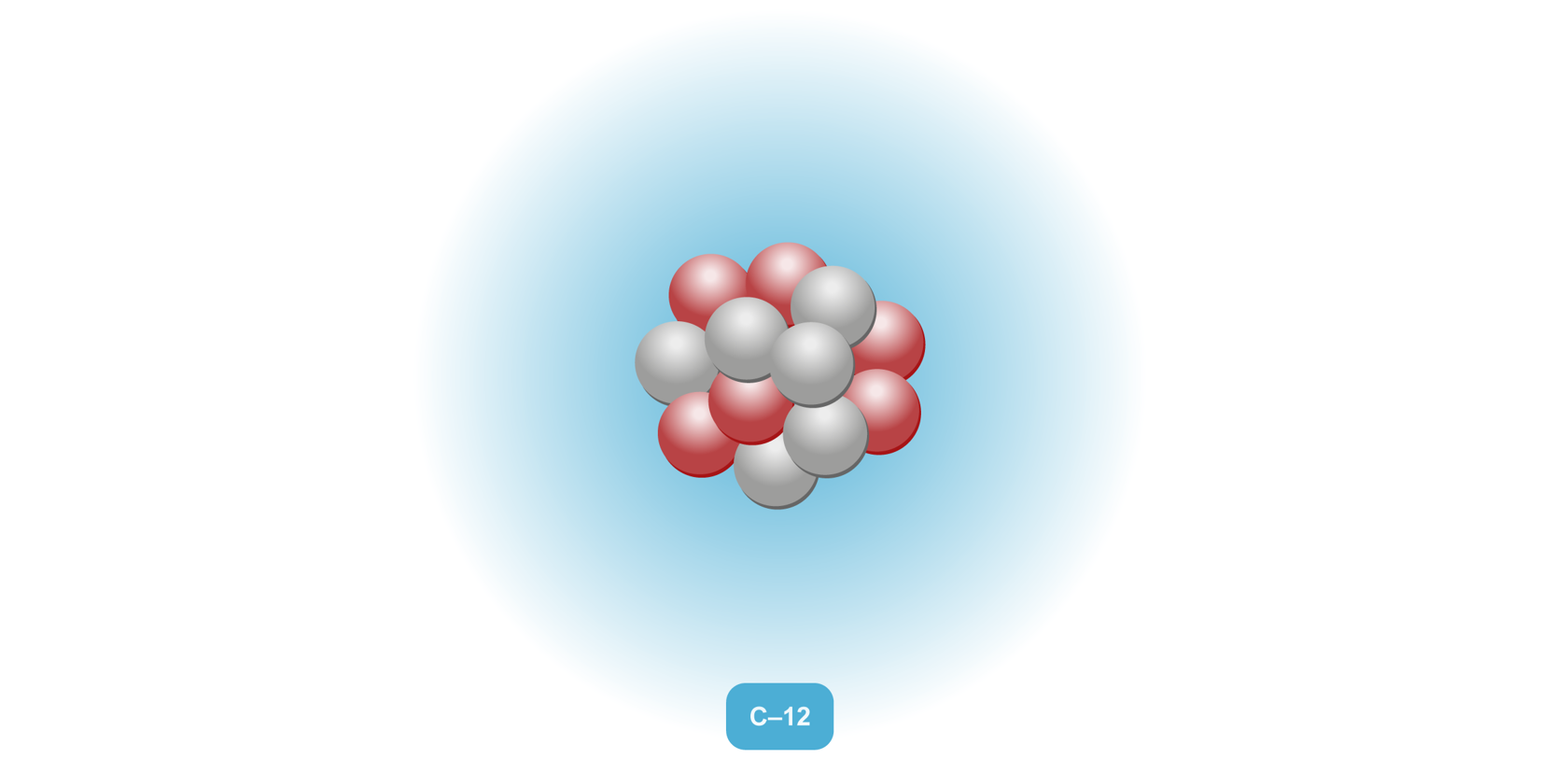 |
The structural components bond to each other with electric attractive forces. The bond’s strength and bonding method can vary (different types of bonds). The bonds result in substances having different characteristics. | 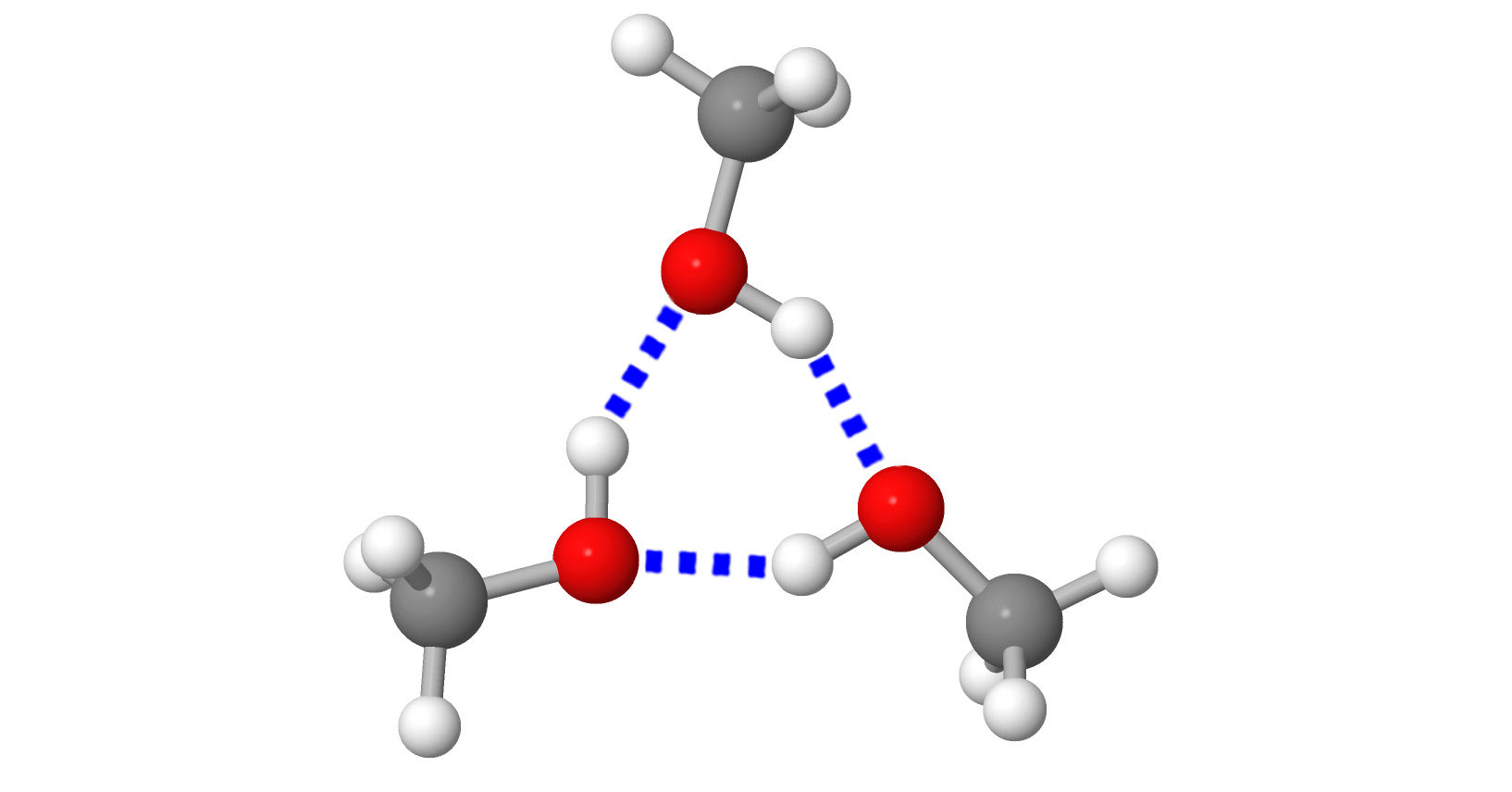 |
Structural components are in constant thermal motion: they move forward, rotate and vibrate. | 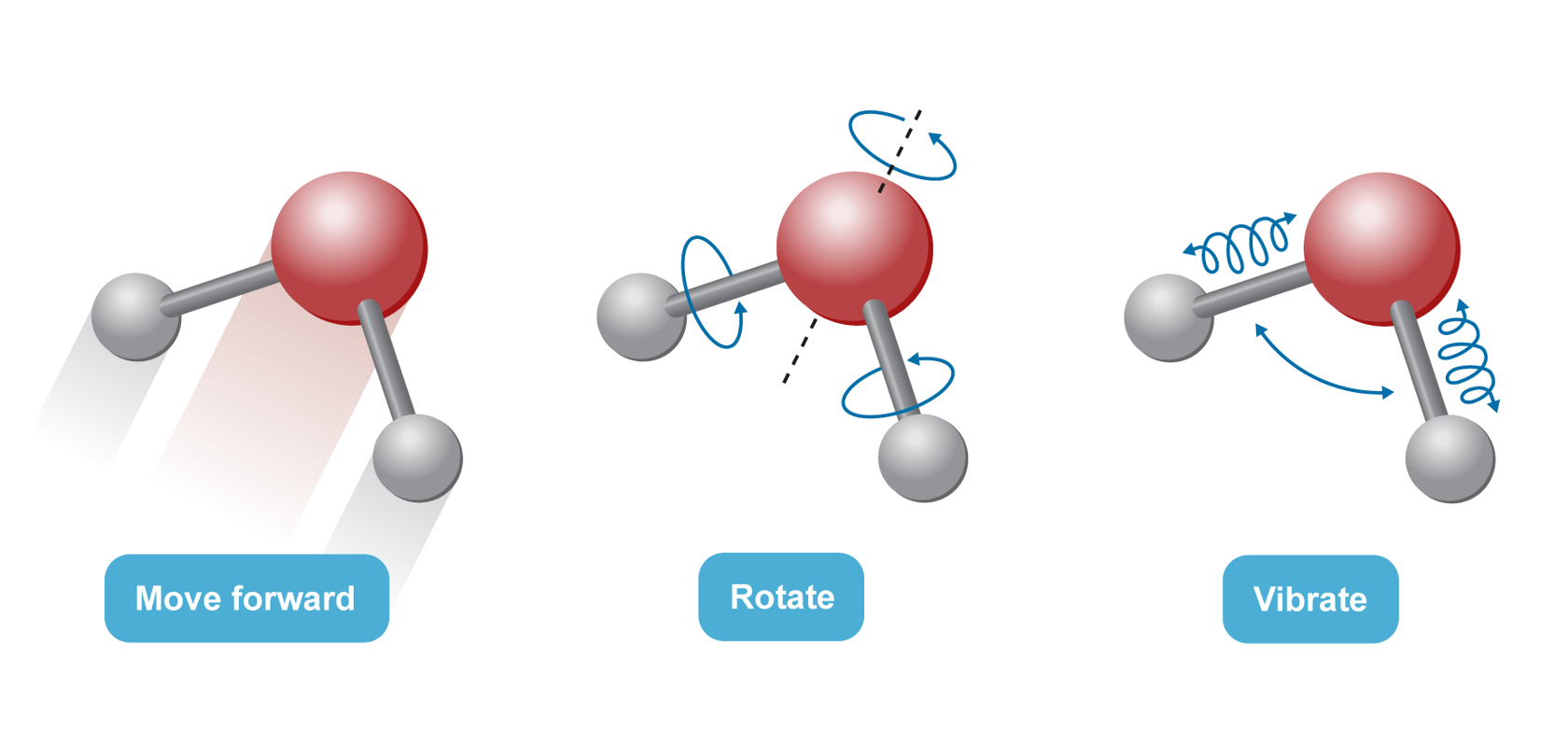 |
When structural components collide, the collision may result in a chemical reaction. In a collision, the components cannot “touch” each other, meaning that they do not get attached to each other physically. | 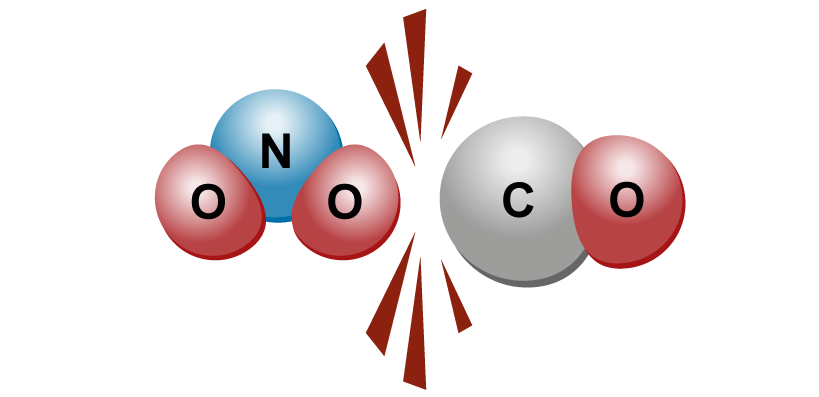 |
Summary about chemical reactions
The table below describes reactions that are divided into the basic categories of inorganic and organic chemistry. The detailed reaction mechanisms and reaction conditions are presented in subsections for each reaction.
Different chemical reactions
Reaction | Inorganic chemistry | Organic chemistry |
Acid-base reactions, proton transfer reactions and neutralisation reactions | In an acid-base reaction, acids transfer a proton to a base (1). In a neutralisation reaction, the reaction between an acid and a base almost always produces salt and water. | The acids are molecules that have an acidic functional group, such as a carboxylic acid or phenol group. Why are phenols acidic? Do some research to find out. An amino group is an alkaline functional group. |
Combustion reactions | A combustion reaction is a reaction where a substance reacts with oxygen. The reaction creates oxides. | When carbon compounds combust, the result is carbon dioxide (incomplete combustion creates carbon monoxide), water and oxides of other substances (e.g. nitrogen oxides). |
Oxidation-reduction (redox) reactions | When an atom loses an electron, it becomes oxidised. When an atom gains an electron, it becomes reduced. An oxidation-reduction reaction involves both reduction and oxidation. The reaction is also called a redox reaction. The reactions involve a change in oxidation numbers, meaning that substances transfer electrons. | Organic substances, such as alcohols and carbonyl compounds, have the following oxidation and reduction reactions: OXIDATION
REDUCTION takes place when the reactions described above are reversed |
Precipitation reactions, ion (exchange) reactions and identification reactions | One example of a precipitation reaction is NaSO4 (aq) + BaCl2 (aq) → BaSO4 (s) + NaCl (aq), where all other substances except for BaSO4 are soluble in water. BaSO4 is only slightly soluble. This is indicated by the (s) symbol. The aforementioned reactions can also be called ion exchange reactions, where the Na and Ba atoms “exchange” the anions joined to them. These reactions are used in inorganic chemistry to identify ions. The reaction creates compounds of a certain colour. | In organic chemistry, identification reactions are the reactions caused by functional groups. However, these reactions are almost always their own reaction groups, such as the addition reactions described in this book. |
Condensation and hydrolysis reactions | Acid-base reactions are also condensation reactions. | In a condensation reaction, two molecules join together, and a small molecule is removed from the point where the molecules combine. The condensed molecule is usually a water molecule. Examples of condensation reactions include esterification and the formation of ether, amides and peptide bonds. A continuous condensation reaction that produces an ever-longer chain is called polycondensation. Hydrolysis is the reverse reaction of condensation. |
Decomposition reactions | E.g. carbonates decomposing into carbon dioxide and metal oxides. | Cracking, which means breaking long carbon chains into smaller sections. |
Reactions that form complexes | The reaction creates a compound consisting of a central atom and ligands that are bonded to it with coordinate covalent bonds. The central atom is usually a transition metal ion. | The formation of chelates is similar to the formation of complexes. Chelates are usually bigger organic compounds that can bind a single metal atom with multiple bonds. This creates a ring-like structure. |
Substitution reaction | Substitution reactions include nitriding (a reaction with nitric acid), sulfonation (a reaction with sulphuric acid) and halogenation. Substitution reactions also occur with aromatic compounds when the hydrogen in a benzene ring is replaced with other elements, for example. | |
Addition reactions | An addition reaction occurs with double and triple bonds. One typical addition reaction is hydrogenation, where hydrogen is added to a compound. | |
Elimination reaction | An elimination reaction produces double-bond compounds. The reaction often removes water. |
These definitions are based on the Brønsted–Lowry acid–base theory. Compare the theory to the Arrhenius definition, which only works with aqueous solutions, or the Lewis definition, which works in broader contexts. According to the Lewis definition, a base transfers an electron pair to the acid. The definition is used when talking about the formation of complexes, for example.