Thermochemistry and thermodynamics
The concept of energy was developed in the early 1800s. Energy is a very important, central concept to chemistry. By researching energy, we can deduce which reactions can and cannot occur, for example. This information can then be used when developing pharmaceutics and other goods.
One key concept related to energy is thermodynamics, which we will cover in this chapter. The concept of thermodynamics was born from observations which showed that heat could transform into other forms of energy, such as chemical energy. In chemistry, thermodynamics studies the energy changes that occur in a reaction. The field that studies thermodynamics is called thermochemistry.
Chemical energy
Energy in chemistry is present as potential energy and kinetic energy. Potential energy is bound in chemical bonds. It affects the interactions between a substance’s structural components. In reactions that break chemical bonds and create new ones, for example, potential energy can be observed as the absorption and release of thermal energy or as radiation energy. Kinetic energy can be observed as the motion of different structural components, meaning the motion of atoms and molecules. They move forward, rotate and vibrate.
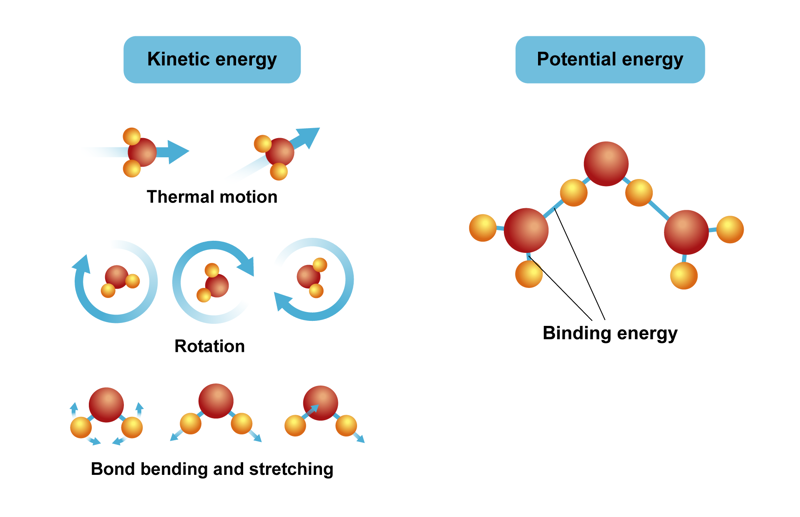
Energy forms in chemical reactions
Energy is absorbed and released in all chemical phenomena. Energy can be converted from one form to another. Conversion can happen several times during the same phenomenon, and the conversion can go both ways. In an explosion, for example, the energy stored in chemical bonds can be converted into electrical, thermal or radiation energy. The energy in food is similarly converted into mechanical energy and thermal energy in the body, which can be observed as muscle movement and body heat.
In this module, we will particularly focus on the thermal energy and electrical energy that is bound and released in reactions. A reaction that releases thermal energy is commonly called an exothermic reaction, while a reaction that absorbs thermal energy is called an endothermic reaction. Combustion is an exothermic reaction, and boiling potatoes in water is an endothermic reaction. Chemically-produced electrical energy is used in batteries and fuel cells, among other things. Electrochemical phenomena are also studied in physics.
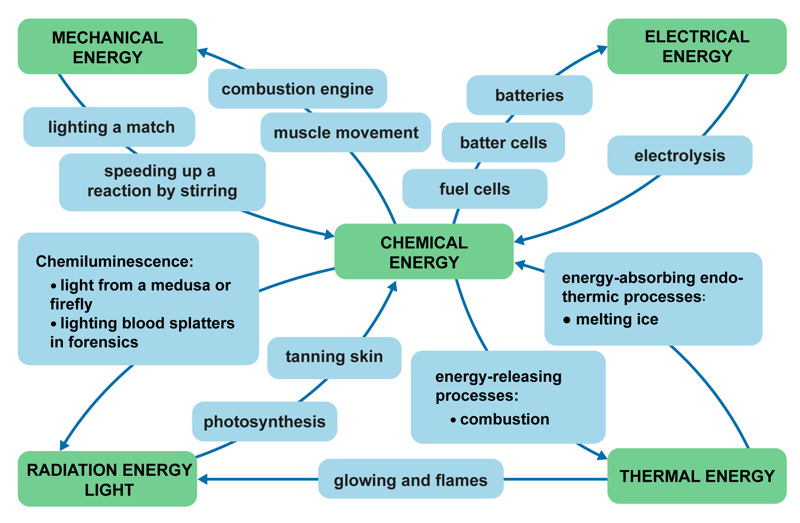
Radiation energy is most commonly observed as light and heat. For example, combustion produces flame, light, colour and temperature phenomena. Magnesium combustion produces a bright white light. The combustion also produces UV radiation, which is harmful to the eyes, so you have to wear safety goggles when looking at the flame. Heating metal salts over a gas burner causes the ions to become excited. When the excited state is released, the bound energy is released as radiation of different wavelengths. We observe the radiation as flames of different colours or as a glow.
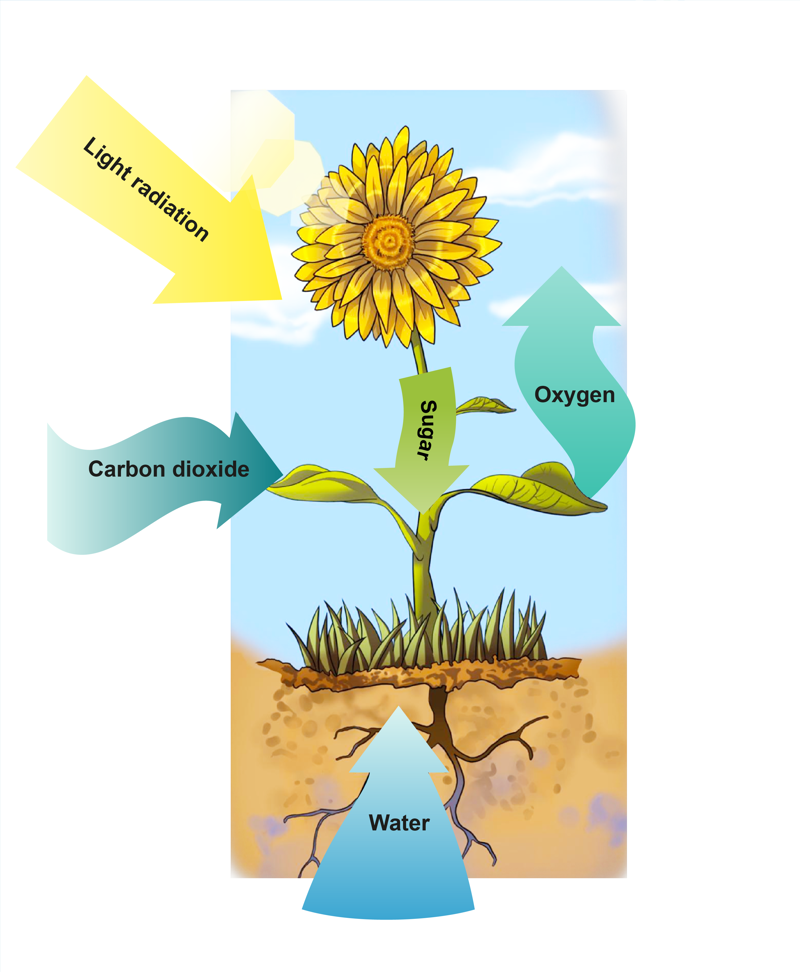
The most vital radiation energy phenomenon for life is photosynthesis. In photosynthesis, plants absorb the radiation energy of sunlight to produce different compounds, such as sugars. Photosynthesis also requires water and carbon dioxide. During your studies, you will most likely learn about radiation in physics and biology as well.
Thermodynamics and reaction kinetics
The foundation of theoretical chemistry research is constructing models based on the positions and movements of atoms and molecules. The models aim to explain the observations we make in the real world. Different descriptive models related to atomic structures and equilibrium states focus on exactly this goal. In both cases, different phenomena are studied from a kinetic perspective, although they can also be studied from a thermodynamic perspective.
Thermodynamics studies the energy changes that occur in natural phenomena, usually for large particle groups. People first became interested in studying thermodynamic phenomena in order to improve steam engines, and thermodynamics later became its own field. In addition to chemistry, thermodynamics plays a central role in physics, biology, engineering and medicine.
In chemistry, thermodynamics studies the energy bound to the bonds between molecules and compounds, for example. This type of energy is called bond energy. Chemical reactions involve bonds breaking and new ones being formed, and that always involves energy changes. Research aims to develop the rules related to energy changes that can be used to predict which reactions are and are not possible. The models created in thermodynamics can be used to predict which reactions and spontaneous, meaning that they occur on their own, and which reactions are nonspontaneous, meaning that they only occur when the reaction conditions (e.g. heat, pressure or acidity) are changed or when a catalyst is used. The field of chemistry related to energy changes is called thermochemistry.
Understanding thermodynamics and reaction kinetics is particularly important in the chemical industry. As an example, thermodynamics and reaction kinetics can be used to optimise industrial processes, making them as efficient and clean as possible. Biochemistry requires information about enzyme catalysation mechanisms to better understand how cells function and to develop more powerful and cleaner production methods for industries like the food industry. The pharmaceutical industry needs information on how quickly medical substances decompose and how much time they need to take effect in the body.
Internal energy
The first law of thermodynamics is the conservation of energy. The amount of substance and energy in a chemical phenomenon remains the same before the reaction and after it. Energy can be converted from one form to another, but no new energy is created, and no energy is lost.
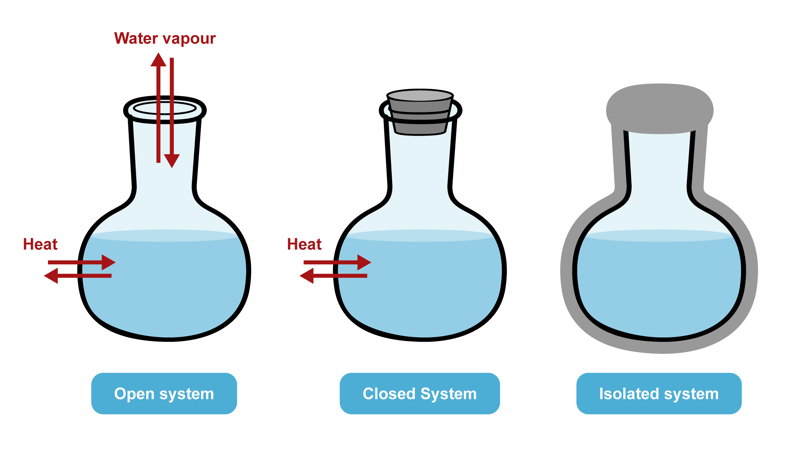
The entities that are studied in thermodynamics are called systems. A system can be formed by a certain amount of a specific substance, for example. An open system can exchange both energy and matter with its surroundings. A closed system can exchange energy but not matter with its surroundings. An isolated system cannot exchange either energy or matter with its surroundings.
A homogenous system only contains one phase. For example, gas or clean liquid will form a homogenous system. A heterogenous system contains two or more phases. Water and water vapour placed in a closed container form a heterogenous system containing a liquid and gas phase. A phase is always thought of as homogenous. Gases can mix with each other at any ratio, so the system only has one phase, unless there is a wall between the gases. Solid and liquid substances can have several phases present in them at the same time.
When a system changes from state A to state B, it experiences a change of state. The way in which the change happens is called a process. When a system changes from state A to state B, its properties also always change. The way in which the change of state happens, the process, can vary a lot.
The system is in a thermodynamic equilibrium if its observable properties do not change even after a long time. When a system is in a thermodynamic equilibrium, it has achieved thermal equilibrium, chemical equilibrium and mechanical equilibrium. The sum of all energies in a system is considered the system’s internal energy, . It is difficult to determine an absolute value for internal energy, because it depends on complex interactions between particles, and thermodynamics studies large particle groups. Fortunately, we only need to know the change in internal energy for thermodynamics. Measuring this change is much easier. The first law of thermodynamics means that a system’s internal energy only depends on the state that the system is in – not on how the system entered its current state. For example, a soft drink bottle will always have the same amount of internal energy in NTP conditions. It does not matter how the soft drink was made and how the drink’s current state was achieved
For water molecules, the warmer the water, the more kinetic energy the molecules have. The thermal motion of molecules consists of many kinds of motion. If we assume that a water molecule is as small as possible, it can move directly forward and change its direction when it collides with something. Water molecules also rotate and their bonds vibrate. During bond vibration, the distances between atoms and the angles between structural components are constantly changing. In addition to kinetic energy, the covalent bonds of water molecules and the bonds between molecules have potential energy due to electrical interactions. The sum of all energies mentioned above is the internal energy of water . When a system changes from state A to state B, the change in internal energy is . When we heat water from 20 °C to 30 °C, for example, the change in internal energy only depends on the amount of energy that was transferred into the system.
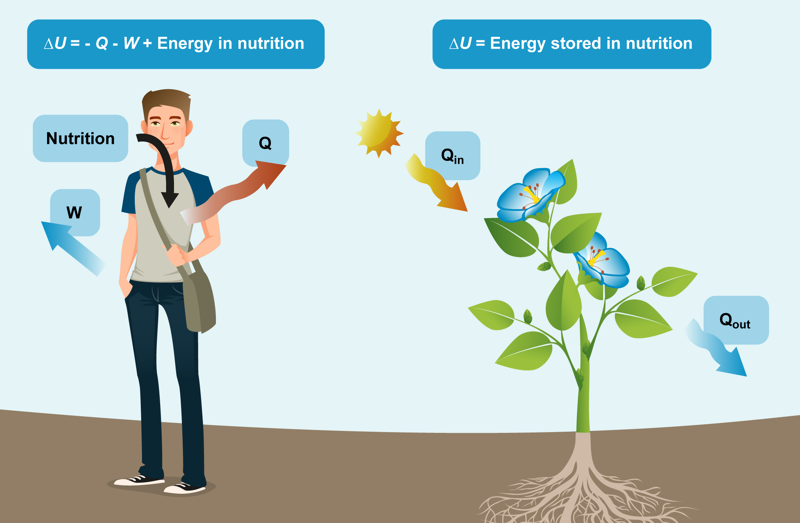
If the amount of internal energy increases,
