Structure and classification of hydrocarbons
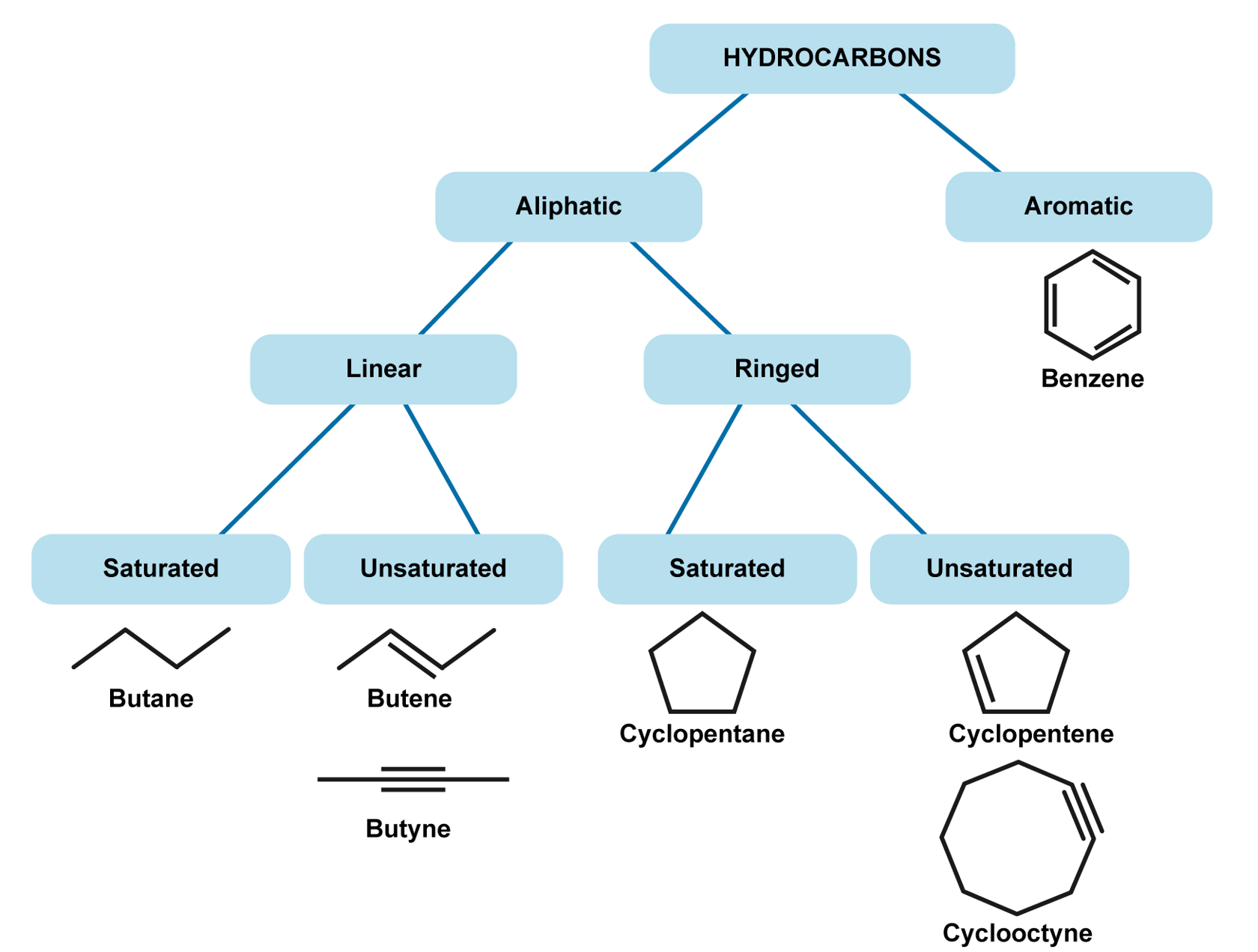
As their name suggests, hydrocarbons consist of hydrogen and carbon. Hydrocarbons are grouped into aliphatic and aromatic hydrocarbons. Aliphatic hydrocarbons are joined together in straight chains or in rings, with the exception of benzene. These can be further divided into saturated and non-saturated hydrocarbons. In a saturated hydrocarbon, there are only single bonds between carbon atoms. In an unsaturated compound, there are one or more double or triple bonds between the carbon atoms. Saturated hydrocarbons include e.g. alkanes and unsaturated hydrocarbons include alkenes and alkynes. Hydrocarbons that contain a benzene ring are classed as aromatic compounds. They are termed as aromatic because many aromatic compounds have a distinct smell, or an aroma. Aromatic compounds have monocyclic, bicyclic, and polycyclic structures.
Hydrocarbon reactions
Combustion
Hydrocarbons can participate in many chemical reactions, the most familiar of which is combustion. When a hydrocarbon reacts with oxygen, it produces lots of energy that is usually observed as heat and light. When a hydrocarbon undergoes a complete combustion reaction, only carbon dioxide and water are formed, while in incomplete combustion, other products may form, such as carbon monoxide, CO, or soot, C. In hydrocarbon combustion reactions, we aim for combustion that is as complete as possible, because material and energy are lost in incomplete combustion. The products of incomplete combustion are also more hazardous to humans and animals when inhaled than the products of complete combustion. Incomplete combustion can be identified by a visible flame. The products of complete combustion are odourless and colourless gases, which are not visible.

Other hydrocarbon reactions
Carbon compounds, along with hydrocarbons, are mainly dealt with in module KE4. The reactions of carbon and oxygen compounds will be examined later in connection with alcohols.
